Q1. Which is a state of a substance from which a phase change occurs without a change of pressure or temperature?a. pure state
b. phase state
c. saturation state
d. critical state
View Answer / Hide Answer ANSWER: c. saturation state
Q2. What is the saturated solid state?a. a state at which solid can change into liquid at constant pressure but changing temperature
b. a state at which solid can change into liquid at constant temperature but change in pressure
c. a state at which solid can change into liquid at constant pressure and temperature
d. none of the above
View Answer / Hide Answer ANSWER: c. a state at which solid can change into liquid at constant pressure and temperature
Q3. What is the state, at which saturated liquid line with respect to vaporisation and saturated vapour line on p-v diagram of pure substance, meet called?a. saturation state
b. critical state
c. vaporisation state
d. superheated vapour state
View Answer / Hide Answer ANSWER: b. critical state
Q4. What is the area highlighted between the two saturated liquid lines in the following p-v diagram of pure substance called?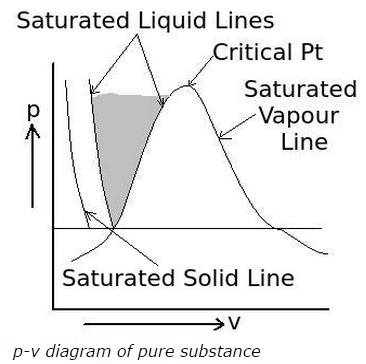
a. saturated liquid region
b. compressed liquid region
c. unsaturated solid region
d. solid-liquid region
View Answer / Hide Answer ANSWER: b. compressed liquid region
Q5. What is the area highlighted between the saturated solid line and the saturated liquid line with respect to solidification in the following p-v diagram of pure substances called?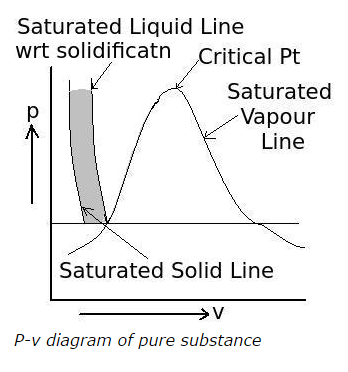
a. solid region
b. solidified liquid region
c. solid-liquid mixture region
d. liquid region
View Answer / Hide Answer ANSWER: c. solid-liquid mixture region
Q6. The temperature of a substance at which the vapour pressure is equal to 760 mm Hg is called asa. normal vapour point
b. normal boiling point
c. normal pressure point
d. none of the above
View Answer / Hide Answer ANSWER: b. normal boiling point
Q7. The temperature at which a pure liquid transforms into vapour at constant pressure is called asa. vaporisation temperature
b. normal temperature
c. saturation temperature
d. none of the above
View Answer / Hide Answer ANSWER: c. saturation temperature